Environmental Monitoring: Testing Facilities for Contamination in Manufacturing
When a product leaves a manufacturing facility, it should be safe. No one wants to find mold in their medicine, Listeria in their deli meat, or metal particles in their lotion. But how do you know your facility isn’t quietly letting contamination slip through? That’s where environmental monitoring comes in. It’s not just a checklist. It’s the frontline defense against product recalls, consumer illness, and regulatory shutdowns.
What Environmental Monitoring Actually Does
Environmental monitoring isn’t about testing the final product. It’s about testing the air, surfaces, water, and equipment before the product even touches them. Think of it like checking your kitchen counters, sink, and cutting boards before you prep dinner - except in a pharmaceutical plant or food factory, one missed spot can mean thousands of contaminated units.
The goal is simple: catch contamination before it becomes a problem. The CDC says environmental sampling is one of the most reliable ways to verify that microbial hazards are under control. The FDA and EMA don’t just recommend it - they require it. In 2023, the FDA made it clear: if you’re making drugs, food, or cosmetics, you need a documented environmental monitoring program. No exceptions.
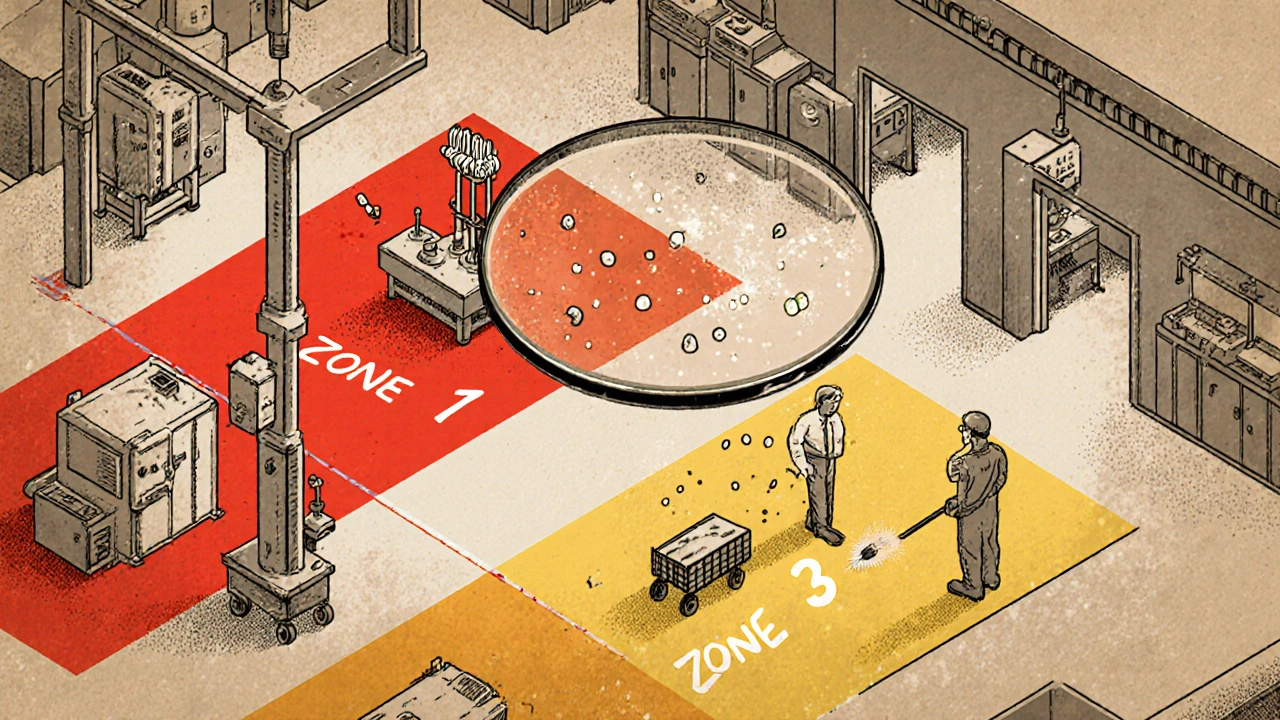
The Zone System: Not All Surfaces Are Created Equal
Not every surface in your facility carries the same risk. That’s why every serious facility uses the Zone classification system. It divides your space into four risk levels:
- Zone 1: Direct contact with the product. Think conveyor belts, filling nozzles, mixing blades, slicers, and packaging surfaces. This is ground zero. If it’s dirty here, your product is compromised.
- Zone 2: Surfaces near Zone 1. Equipment housings, refrigeration units, nearby walls, and carts that roll close to production. These surfaces can kick up dust or splash contaminants onto Zone 1.
- Zone 3: Areas near production but not directly involved. Floors, overhead pipes, forklifts, and storage racks. Sounds low risk? In a 2010-2013 study by PPD Laboratories, floors were the source of 62% of all contamination events - more than any other zone.
- Zone 4: The rest. Break rooms, offices, hallways, entrances. Still monitored, but less frequently. These areas help track if contamination is spreading from inside to outside.
Here’s the catch: Zone 1 gets tested daily or weekly. Zone 2 gets weekly or monthly checks. Zones 3 and 4? Monthly to quarterly. But if you skip Zone 3, you’re ignoring the real source of most problems. One facility thought overhead pipes were safe - until condensation dripped onto a product line, introducing mold. Now they test those pipes like Zone 1.
How Testing Works: Tools and Methods
Testing isn’t just swabbing surfaces and waiting. Different contaminants need different tools:
- Microbiological swabs: Used on Zone 1 and 2 surfaces. Sterile sponges or swabs collect samples from slicers, mixers, and drains. Lab results take 24-72 hours to identify bacteria like Listeria or Salmonella.
- Air samplers: Liquid impingers and solid impactors pull air through a device, trapping microbes or particles. Results are measured in CFU/m³ (colony-forming units per cubic meter). Critical cleanrooms in pharma need continuous monitoring - even tiny spikes can trigger shutdowns.
- ATP testing: This isn’t a microbiological test. It detects adenosine triphosphate, a molecule found in all living cells. It gives results in seconds. Facilities using ATP see 32% faster turnaround between production runs because they don’t wait for lab results. It’s a quick check - not a replacement, but a filter.
- Water testing: Pharma facilities test purified water with TOC (total organic carbon) and conductivity to meet USP <645> standards. Food plants check municipal water against EPA rules.
- ICP and chromatography: For metals or chemical residues. Inductively Coupled Plasma (ICP) finds trace metals. HPLC and GC find specific chemicals like cleaning agent residues.
Here’s what most facilities get wrong: they mix up the tools. ATP is great for sanitation checks, but it won’t tell you if you have Listeria. Swabs are slow but definitive. You need both.
Industry Differences: Pharma vs. Food vs. Cosmetics
Not all industries monitor the same way. The rules change depending on what you make.
| Aspect | Pharmaceutical | Food (RTE) | Cosmetics |
|---|---|---|---|
| Primary Target | Endotoxins, fungi, airborne particles | Listeria monocytogenes, Salmonella | Molds, yeasts, Pseudomonas |
| Air Quality Standard | ISO Class 5 (EU Grade B) - continuous monitoring | No formal air class - focus on microbial air counts | ISO Class 7-8 - occasional monitoring |
| Water Testing | USP <645> - TOC and conductivity | EPA standards - no TOC required | USP <61> for microbial limits |
| Regulatory Driver | EU GMP Annex 1 (2023) | USDA 9 CFR Part 430 (Listeria Rule) | US FDA Cosmetic GMP (2023) |
| Testing Frequency (Zone 1) | Daily to twice daily | Weekly (mandatory for RTE) | Weekly to biweekly |
Pharma is the strictest. Airborne particles are monitored 24/7. Food focuses on pathogens - especially Listeria in ready-to-eat products. Cosmetics are catching up, but many still treat monitoring as optional. That’s changing. In 2023, the FDA updated its cosmetic GMP rules to require environmental monitoring for products with water-based ingredients.
What Goes Wrong: The Common Mistakes
Most facilities have good intentions. But here’s where they fail:
- Inconsistent zone classification: One manager thinks a pipe is Zone 2. Another thinks it’s Zone 1 because it drips. No standard means no control.
- Bad sampling technique: Swabs aren’t sterile. Air samplers aren’t cleaned between uses. Samples get contaminated before they’re even tested.
- Ignoring data integration: ATP results, microbiology reports, and allergen tests sit in separate spreadsheets. You can’t see the full picture.
- Understaffing: A medium-sized food plant needs 2-3 full-time people just to run monitoring. Many small facilities skip this - and pay later.
- Training gaps: The FDA says staff need at least 40 hours of hands-on training before sampling. Most get 2 hours and a manual.
According to the IDFA’s 2020 survey, 68% of facilities struggle with sampling technique. That’s not a technology problem - it’s a people problem.
What’s New: Tech and Trends
Environmental monitoring isn’t stuck in the 1990s. New tools are changing the game:
- Next-generation sequencing (NGS): Instead of waiting 3 days to identify a microbe, NGS can tell you the exact species in under 24 hours. The FDA is pushing for this in 2025.
- AI-powered analytics: Software now tracks trends in contamination data. If Listeria pops up in Zone 3 every Tuesday, it flags a pattern - maybe a leaky pipe or a cleaning shift issue.
- Real-time monitoring: EU GMP Annex 1 (2023) now requires continuous data logging in critical areas. No more manual logs. Sensors send data straight to a dashboard.
- Antimicrobial resistance tracking: The CDC found 19% of Listeria strains from food plants now resist multiple antibiotics. Monitoring isn’t just about contamination - it’s about public health threats.
By 2027, 38% of environmental monitoring systems will use AI, up from 12% in 2022. The market is growing fast - from $7.2 billion in 2022 to $12.5 billion by 2027. That’s because companies are realizing: prevention is cheaper than recall.
Why It Matters: The Real Cost of Failure
A single contamination event can cost millions. The USDA estimates foodborne illnesses cost the U.S. $77.7 billion a year. Most of those could have been prevented with better environmental monitoring.
Think about it: a recall means lost product, lost time, lost trust. The FDA can shut you down. A single Listeria outbreak in a deli meat plant led to 10 deaths and a $100 million recall in 2022. That facility didn’t test Zone 3 floors regularly. They thought it was low risk.
Meanwhile, companies with strong monitoring programs report 90% fewer deviations during FDA inspections. Their audits are smoother. Their staff are more confident. Their products are safer.
Getting Started: What You Need to Do
If you’re starting from scratch, here’s your roadmap:
- Map your facility. Define Zones 1-4 based on actual product contact and risk - not guesswork.
- Choose your tests. Use ATP for quick sanitation checks. Use swabs and air samplers for microbial confirmation.
- Set frequencies. Zone 1: daily/weekly. Zone 2: weekly/monthly. Zones 3-4: monthly.
- Train your team. 40 hours of hands-on training isn’t optional. It’s the law for FDA-regulated facilities.
- Integrate your data. Use software that brings together ATP, microbiology, and environmental logs in one place.
- Review monthly. Look for trends. If a spot keeps testing positive, fix the root cause - not just the swab.
You don’t need to spend $500,000 on a cleanroom. You need a smart, consistent, documented system. That’s what regulators look for. That’s what keeps your product safe.
What is the main purpose of environmental monitoring in manufacturing?
The main purpose is to detect and control contamination - like bacteria, mold, or chemical residues - in the production environment before it gets into the product. It’s a preventive step, not a reaction. By testing air, surfaces, and water regularly, facilities can catch problems early, avoid product recalls, and meet regulatory standards.
How often should environmental testing be done?
Frequency depends on the zone and industry. Zone 1 (direct product contact) is tested daily or weekly. Zone 2 (near contact) is tested weekly to monthly. Zones 3 and 4 (remote areas) are tested monthly to quarterly. For FDA-regulated ready-to-eat food facilities, Zone 1 must be tested for Listeria at least once a week. Pharmaceutical cleanrooms require continuous air monitoring.
What’s the difference between ATP testing and microbiological testing?
ATP testing detects organic residue from all living cells - it gives a quick readout in seconds, showing if a surface is clean. But it doesn’t tell you what kind of microbe is there. Microbiological testing (like swabs sent to a lab) takes 24-72 hours but identifies specific pathogens like Listeria or Salmonella. Use ATP for fast checks. Use microbiological tests for confirmation and compliance.
Why do floors cause so many contamination events?
Floors are high-traffic areas where dirt, water, and debris accumulate. They’re often overlooked because they don’t touch the product directly. But people, carts, and equipment track contaminants from Zone 3 to Zone 1. A 2010-2013 study found floors caused 62% of all contamination events - more than any other surface. That’s why they’re critical to monitor, even if they’re labeled Zone 3.
Can small manufacturers afford environmental monitoring?
Yes, but it requires smart prioritization. Small facilities don’t need full cleanrooms. They can start with Zone 1 swabs, ATP testing, and weekly floor checks. The average small food plant spends $15,000-$25,000 a year on monitoring - less than the cost of one recall. Many use outsourced labs for testing and train one employee to handle sampling. The key is consistency, not complexity.
What happens if a facility fails an environmental monitoring inspection?
The FDA or USDA can issue a warning letter, halt production, or even initiate a recall. Repeated failures can lead to facility shutdowns. In 2023, the FDA increased inspection frequency for high-risk food facilities, and environmental monitoring violations are now among the top reasons for regulatory action. It’s not a fine - it’s a stop order.





14 Comments
Nick Naylor
November 20, 2025 at 12:14
Environmental monitoring isn't optional-it's a regulatory mandate with teeth. Zone 1 swabs? Daily. Air samplers? Continuous in ISO Class 5. ATP? Great for triage, but it's not a substitute for microbiological validation. If you're skimping on sampling frequency or training, you're playing Russian roulette with FDA warning letters. The 2023 EU GMP Annex 1 update? Non-negotiable. And don't even think about ignoring Zone 3 floors-62% of contamination events originate there. This isn't theory-it's compliance. Fail, and your facility gets shut down. Period.
Brianna Groleau
November 20, 2025 at 19:43
I just want to say how deeply moved I am by how much care goes into keeping our food and medicine safe-people don't realize the quiet heroes in white coats swabbing floors at 3 a.m., the lab techs waiting 72 hours for results, the engineers designing air systems that whisper instead of roar. This isn't just science-it's love. It's the difference between a child taking medicine without fear, and a mother reading a recall notice with tears in her eyes. Every swab, every sensor, every data log is a silent promise: 'We won't let you down.' And we shouldn't take that for granted.
Rusty Thomas
November 21, 2025 at 14:43
Okay, but let’s be real-how many of these 'strict' facilities are just doing it for the audit? I’ve seen plants where the Zone 1 swabs are taken by the same guy who cleans the bathroom. The air samplers? Collected once a month and 'accidentally' left in the break room. And ATP? Used as a prop during inspections. The FDA doesn’t have eyes everywhere, and guess what? People game the system. That’s why recalls still happen. It’s not the tech-it’s the people. And if you think your 'compliant' facility is clean, you’re just deluding yourself. 😒
Sarah Swiatek
November 21, 2025 at 23:32
It’s funny how we treat contamination like it’s a glitch in the system, when really, it’s a symptom of a system designed to optimize output, not safety. We build factories to move product fast, then slap on monitoring like a Band-Aid. We train staff for two hours and expect them to become microbiologists. We cut corners on Zone 3 because it’s 'not direct contact'-as if contamination reads a flowchart. The real tragedy isn’t the Listeria outbreaks-it’s that we only fix things after someone dies. We don’t need more sensors-we need humility. We need to admit that safety isn’t a checklist. It’s a culture. And culture doesn’t scale. It must be nurtured. Every. Single. Day.
Rebecca Cosenza
November 23, 2025 at 19:37
If you’re not testing Zone 1 daily, you’re negligent. End of story.
Cinkoon Marketing
November 24, 2025 at 21:18
Hey, we’re a small cosmetic brand in Canada and we started with just ATP swabs on our filling nozzles and weekly floor checks. Costs us like $18k/year. No fancy AI. No full-time staff. Just consistency. FDA didn’t even blink during our last inspection. You don’t need to be pharma-tier to be safe. Just be smart.
Bill Camp
November 25, 2025 at 13:35
Look, I’ve worked in three different food plants. The ones that think they’re doing it right? They’re the ones getting shut down. The ones who don’t talk about it? The quiet ones with the clean logs and the trained staff? They’re the ones still open. Monitoring isn’t about bragging rights. It’s about not being on the news. And if you’re reading this and you’re not doing Zone 3? You’re already on the list.
Lemmy Coco
November 26, 2025 at 04:27
just read this whole thing and wow. i never thought about how the floors are the biggest culprit. i always thought it was the machines. also, the part about training-40 hours? i work at a small facility and our 'training' was a 20-min video. that’s not enough. i’m gonna talk to my manager. thanks for the info.
rob lafata
November 27, 2025 at 00:01
You think this is about safety? Nah. It’s about liability. Every regulation, every swab, every sensor-designed to protect corporations from lawsuits, not people. The FDA doesn’t care if you have mold in your lotion-they care if you get sued. And if you’re dumb enough to believe this is about health, you’re the exact kind of sucker who buys 'natural' skincare labeled 'tested' while the lab report says 'found 12,000 CFU of Pseudomonas.' Wake up. This isn’t science. It’s PR with a microscope.
Matthew McCraney
November 28, 2025 at 20:15
They’re lying. All of it. Environmental monitoring? A scam. The real contamination comes from the water supply-corporate water treatment plants are rigged. They add chemicals to mask the pathogens so the swabs come back clean. And the 'AI analytics'? Just a front. The same people who run the factories own the labs. The CDC? Controlled. The FDA? Bought. That’s why recalls are so rare-because they’re buried. You think your medicine is safe? It’s not. They’re feeding you poison and calling it 'compliance.'
serge jane
November 29, 2025 at 05:40
There’s something profoundly human about this whole process. We’re not just checking for microbes-we’re checking for our own negligence. Every swab is a mirror. Every positive result is a question: Did we care enough? Did we train enough? Did we listen? We build these systems to protect the product, but the product is just the vessel. The real thing we’re protecting is trust. And trust isn’t measured in CFU per cubic meter. It’s measured in how many people sleep peacefully at night because they believe someone, somewhere, was paying attention.
Dave Wooldridge
November 29, 2025 at 11:09
Next-gen sequencing? AI? Please. They’re just using tech to distract from the fact that no one’s fixing the root cause. The real problem? The machines are old, the pipes leak, and the janitors are paid minimum wage. You can’t algorithm your way out of a broken system. They’re putting sensors on the walls while the foundation rots. And when the next outbreak happens? They’ll blame 'human error.' Always human error. Never the system that made the human do it.
swatantra kumar
November 30, 2025 at 01:25
Love this breakdown! 🙌 Especially the Zone 3 floor stat-62% is wild! We’re starting ATP testing in our pharma plant in India next month. Small step, but it’s a start. Also, NGS sounds like sci-fi but I’m all in. Let’s get smarter, not just stricter! 💪
robert cardy solano
December 1, 2025 at 02:43
My cousin works in a food plant. He said they started using real-time air monitors last year. Now the whole floor gets shut down if a spike hits. No more 'we’ll check tomorrow.' It’s weird to watch, but kinda cool. Feels like the factory’s alive, breathing. Anyway, good read.