HBV Reactivation: How Biologics and Chemotherapy Trigger Liver Danger - And How to Stop It
Every year, thousands of people undergoing cancer treatment or taking powerful immune drugs for autoimmune diseases face a hidden threat: their own dormant hepatitis B virus waking up and attacking their liver. This isn’t rare. It’s predictable. And it’s preventable - if you know what to look for.
What Exactly Is HBV Reactivation?
HBV reactivation happens when a virus that’s been sleeping in your liver for years suddenly starts multiplying again. This isn’t a new infection. It’s the same virus you’ve carried since childhood - maybe without ever knowing it. People with past or chronic hepatitis B infection often show no symptoms. But when their immune system gets shut down by chemotherapy, biologics, or steroids, the virus takes advantage.
The result? A sudden spike in liver enzymes, jaundice, severe fatigue, and sometimes liver failure. In the worst cases, it’s fatal. Studies show 5-10% of people who experience full-blown reactivation die from it. That’s not a side effect. That’s a preventable medical emergency.
Who’s at Risk? It’s Not Just What You Think
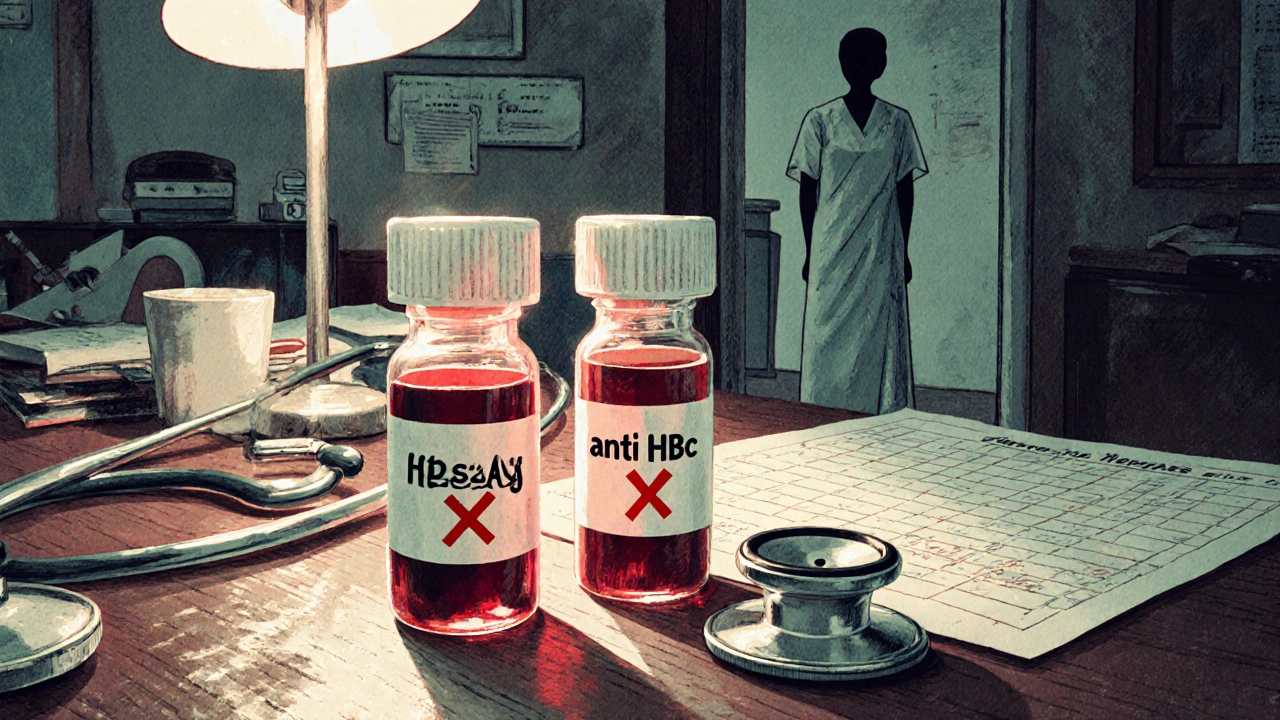
Not everyone with hepatitis B is at equal risk. Your danger level depends on two key blood tests: HBsAg and anti-HBc.
- HBsAg-positive: You have active, chronic hepatitis B. Your risk of reactivation is 20% to 81%, depending on the drug. If you’re getting rituximab for lymphoma? Your risk jumps to nearly 70%.
- HBsAg-negative, anti-HBc-positive: You cleared the virus years ago. Your body still carries traces. This group is often overlooked. But if you’re on high-dose chemo or a stem cell transplant, your reactivation risk is still 1% to 18%. In some cases, it’s higher than people think.
- Both negative: You’ve never had HBV. No risk. No screening needed.
Here’s the catch: many people don’t know their status. In countries with low HBV rates, doctors assume it’s rare. But in the U.S., 1 in 12 Asian Americans, 1 in 20 African immigrants, and 1 in 50 overall have past or chronic infection. Screening isn’t optional - it’s essential.
Which Drugs Are Most Dangerous?
Not all immunosuppressive drugs are created equal. Some are like lighting a match next to gasoline. Others are barely a spark.
High-risk drugs (reactivation risk >10%):
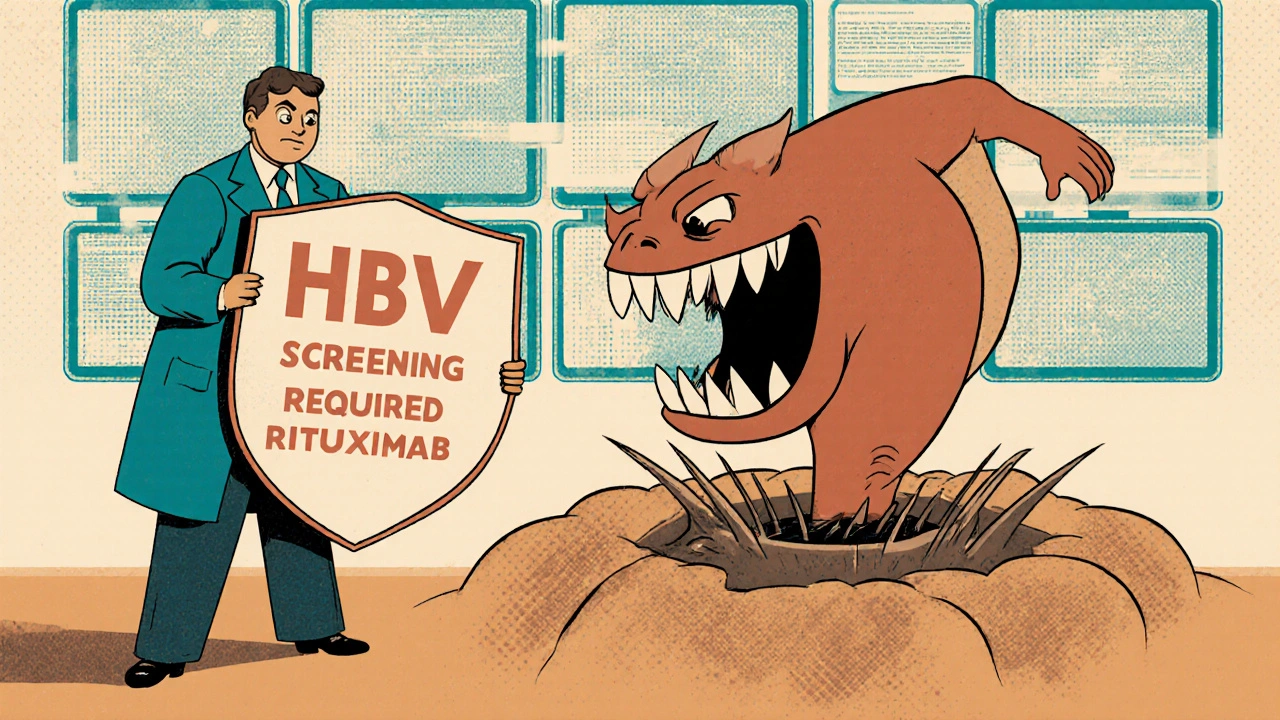
- Rituximab, ofatumumab (anti-CD20 biologics): Used for lymphoma, rheumatoid arthritis. Reactivation risk: 38-73% in HBsAg-positive patients.
- Anthracycline chemo (like doxorubicin): Common in breast cancer and lymphoma. Risk: 25-40%.
- Stem cell transplants: Autologous? 66% risk. Allogeneic? 81% risk. Most reactivations happen in the first year.
- Transarterial chemoembolization (TACE): Used for liver cancer. Reactivation risk: 17-34% - higher than most assume.
- Checkpoint inhibitors (like pembrolizumab): Newer cancer immunotherapies. Risk: 21% in HBsAg-positive patients not on antivirals.
Medium-risk drugs (1-10%):
- Standard chemo without steroids
- TNF-alpha inhibitors (like adalimumab for arthritis)
- Ibrutinib (for leukemia and lymphoma)
Low-risk drugs (<1%):
- Non-TNF biologics (like abatacept)
- Most targeted therapies (like imatinib)
- Low-dose steroids
Don’t assume low risk just because it’s not chemo. Even drugs for arthritis can wake up your virus.
How to Prevent It: Screening and Prophylaxis
The fix is simple: screen everyone before starting immunosuppressive therapy. That means two blood tests - HBsAg and anti-HBc - done at least two weeks before treatment begins.
If you’re HBsAg-positive, you need antiviral prophylaxis. Start it at least one week before your first dose of chemo or biologic. The two most effective drugs are:
- Tenofovir (Viread, Tenvir)
- Entecavir (Baraclude)
These drugs suppress the virus so well that reactivation drops from nearly 50% to under 5%. One study of 1,245 patients found that those on prophylaxis had only a 3.2% reactivation rate. Those without it? 48.7%.
How long do you stay on it? For high-risk drugs like rituximab or stem cell transplants, continue for 6 to 12 months after treatment ends. For checkpoint inhibitors, 6 months may be enough - new data suggests you don’t always need a full year.
For HBsAg-negative, anti-HBc-positive patients? Guidelines are less clear. Some experts recommend prophylaxis only for very high-risk therapies like stem cell transplants. Others suggest it for all. The safest approach? Talk to a liver specialist.
Why Do So Many People Still Get Hurt?
Here’s the uncomfortable truth: most cases of HBV reactivation are avoidable. But they keep happening.
A 2020 survey found only 58% of community oncologists follow screening guidelines. In academic centers? 89%. That gap isn’t about knowledge - it’s about systems. One patient in a case report died after rituximab because no one checked his HBV status. He was 52. He had no symptoms before. He had no warning.
Why does this happen?
- Doctors assume HBV is rare in their patient population.
- Screening isn’t built into the electronic health record.
- Patients are seen by multiple specialists - no one takes responsibility.
- There’s a false belief that if the patient “feels fine,” they’re not at risk.
At UCSF, they fixed this by adding automatic alerts in their EHR. If a patient is flagged for chemo or biologics, the system pops up: “HBV screening required.” Result? Reactivation rates dropped from 12.3% to 1.7% in just five years.
What About the Cost? Is Screening Worth It?
Some argue screening is overkill, especially in low-prevalence areas. But the math doesn’t lie.
Screening costs about $20-$40 per person. Treating a single case of severe reactivation? $50,000 to $150,000. Add ICU stays, liver transplants, lost work time - and the real cost is higher.
Dr. Anna S. Lok, a leading hepatologist, calls antiviral prophylaxis “one of the most cost-effective interventions in modern medicine.” The number needed to treat to prevent one reactivation? Just three. That means for every three people screened and treated, you prevent one life-threatening event.
And the legal risk? In malpractice claims, HBV reactivation makes up 12% of infectious complications in oncology. Hospitals are getting sued for this. It’s not a question of if - it’s a question of when.
What’s Next? The Future of Prevention
Technology is catching up. In late 2023, the FDA is expected to approve the OraQuick HBV rapid test - a finger-prick test that gives results in 20 minutes. Imagine screening in a clinic, not a lab.
Companies like Tempus Labs are now integrating HBV status into genomic cancer profiles. If you’re getting a tumor DNA test, your HBV status comes with it. That’s precision medicine in action.
But tech won’t fix this alone. The real solution is culture change. Every oncologist, rheumatologist, and surgeon needs to treat HBV screening like a pre-op blood test - non-negotiable, automatic, routine.
The tools are here. The guidelines are clear. The evidence is overwhelming. The only thing missing is consistent action.
What Should You Do?
If you’re about to start chemotherapy, a biologic, or any immune-suppressing treatment:
- Ask your doctor: “Have you checked my HBV status?”
- If they say no - insist. Request HBsAg and anti-HBc tests.
- If you test positive for HBsAg, ask for tenofovir or entecavir before treatment starts.
- If you’re anti-HBc-positive, ask if you need prophylaxis based on your treatment.
- Don’t stop antivirals early. Follow your doctor’s timeline - even if you feel fine.
If you’re a healthcare provider:
- Make HBV screening part of your pre-treatment checklist.
- Use your EHR to build automated alerts.
- Refer anti-HBc-positive patients to hepatology if they’re on high-risk therapy.
- Don’t wait for a tragedy to change your practice.
HBV reactivation isn’t a mystery. It’s a missed opportunity. And every case that happens today is one that could have been stopped yesterday.
Can you get HBV reactivation if you’ve been vaccinated?
No. If you were vaccinated against hepatitis B and have never been infected, you won’t have HBV in your body to reactivate. Vaccination creates immunity without leaving behind the virus. Only people with past or current infection - confirmed by HBsAg or anti-HBc blood tests - are at risk.
Do I need to get tested if I’ve had hepatitis B before and it’s resolved?
Yes. Even if you cleared the virus years ago and have no symptoms, your liver still holds traces of HBV. If you take strong immunosuppressants like rituximab or chemotherapy, that virus can wake up. The risk is lower than for chronic carriers, but it’s real - up to 18% in high-risk treatments. Screening and sometimes prophylaxis are still needed.
How long do I need to take antivirals after my treatment ends?
For high-risk treatments like rituximab, stem cell transplants, or anthracycline chemo, take antivirals for 6 to 12 months after your last dose. For checkpoint inhibitors or moderate-risk therapies, 6 months may be enough. Never stop early without talking to your doctor. Stopping too soon can trigger reactivation even after treatment ends.
Can HBV reactivation happen with radiation therapy?
Yes. Radiation therapy, especially for liver cancer, carries a 14% reactivation risk in people who are HBsAg-negative but anti-HBc-positive. This is often overlooked because radiation isn’t a drug. But it still suppresses the immune system locally and sometimes systemically. Screening is recommended before starting radiation if you have any history of HBV exposure.
Are there side effects from taking tenofovir or entecavir long-term?
Both drugs are very safe for long-term use. Tenofovir can rarely affect kidney function or bone density, so doctors monitor these with blood tests. Entecavir has even fewer side effects. The risks of these drugs are far lower than the risks of liver failure from reactivation. Most people tolerate them well and experience no issues.
What if I can’t afford the antiviral drugs?
Generic versions of tenofovir and entecavir are widely available and cost under $10 per month in many countries. In the U.S., patient assistance programs from Gilead and other manufacturers can cover the full cost. Never skip screening or prophylaxis because of cost - the financial and health cost of reactivation is far higher.
Can HBV reactivation happen during or after biologic therapy for arthritis?
Yes. Biologics like rituximab, infliximab, and adalimumab are linked to HBV reactivation. Even if you’re being treated for rheumatoid arthritis and feel healthy, your immune system is suppressed. If you’ve ever had hepatitis B - even decades ago - you’re at risk. Screening before starting any biologic is standard of care.
Why do some doctors still not screen for HBV before treatment?
Some doctors assume HBV is rare in their region or patient group. Others don’t know the guidelines or aren’t trained in liver disease. Many don’t have systems in place to flag patients. But guidelines from AASLD, EASL, and IDSA are clear: screen everyone. The consequences of skipping it are too severe to ignore.





13 Comments
kanishetti anusha
November 16, 2025 at 12:50
This is such an important topic that gets buried under the noise of flashy cancer treatments. I work in oncology nursing in India, and I’ve seen too many patients come in with liver failure after starting chemo-no one ever checked their HBV status. It’s not just about the drugs; it’s about systemic neglect. Screening should be mandatory before any immunosuppressive therapy, period.
And to those who say, 'But it’s rare here'-it’s not rare in our communities. One in twelve Asian Americans? In India, it’s even higher in certain regions. We need better awareness, not just better drugs.
Stop assuming. Start testing.
Thank you for writing this.
roy bradfield
November 18, 2025 at 02:32
Let me tell you something they don’t want you to know. The pharmaceutical companies don’t screen for HBV because if they did, they’d have to pay for antivirals for millions of people. That’s not profit-driven. That’s a liability. And who gets stuck with the bill? You. The patient. The system is designed to let you get sick first, then charge you triple for the fix.
They know rituximab causes reactivation. They’ve known for 15 years. But they keep selling it like it’s a miracle cure. Meanwhile, your liver turns to mush and your family gets a bill for $400,000. This isn’t medicine. It’s a business model built on ignorance and fear.
And don’t even get me started on how they classify 'low-risk' drugs. Last month, my cousin got hepatitis from adalimumab. They said 'low-risk.' He’s on the transplant list now. Low-risk? More like low-ethics.
Patrick Merk
November 18, 2025 at 13:48
Really appreciate this breakdown-it’s the kind of thing I wish I’d known before my aunt started her RA treatment. We just assumed 'no symptoms = no problem.' Turns out, her anti-HBc was positive, and they almost missed it. She’s on entecavir now and doing fine, but it was terrifying.
Also, the point about TACE being high-risk? That’s wild. I’m a radiologist in Dublin, and we never flagged that in our protocols until last year. It’s insane how much we overlook when the virus is 'inactive.'
Bottom line: if you’re immunosuppressed and haven’t been screened, ask for HBsAg and anti-HBc. It’s a two-minute blood test. Could save your life.
Liam Dunne
November 20, 2025 at 07:42
Just had a patient last week-62, lymphoma, HBsAg positive, never knew. Got rituximab without antivirals. Ended up in ICU. We caught it late. He’s stable now, but barely.
Doctors need to stop thinking 'HBV is a third-world problem.' It’s everywhere. Even in rural Maine, I’ve seen it. The data doesn’t lie. If you’re giving chemo or biologics, screen. Period. No exceptions.
And if you’re a patient? Don’t be shy. Ask. Even if your doctor says 'you’re fine.' Get the bloodwork. It’s not paranoia. It’s survival.
Vera Wayne
November 21, 2025 at 14:50
Thank you, thank you, thank you-for writing this, for sharing this, for not letting this slide under the radar!!!
I’ve been pushing for universal HBV screening in my clinic for years, and I’ve been told it's 'not cost-effective' and 'too much paperwork.' But when someone dies because we didn’t check a simple blood test… what’s the cost then?
Let’s make this standard. Let’s make it mandatory. Let’s stop pretending this is optional.
Also, I printed this out and gave it to my entire team. Everyone needs to read it.
Rodney Keats
November 23, 2025 at 08:46
Oh wow, so now we’re supposed to screen everyone for a virus that’s been sleeping for decades… just in case some drug wakes it up? What’s next? Do we test for the flu before giving aspirin?
Maybe instead of turning every treatment into a full medical detective show, we should just stop giving people drugs that kill them. Oh wait-that’s not profitable either.
So yeah, let’s screen everyone. Because why not add another 100 forms to the intake packet? Who cares if the patient dies of liver failure? At least their chart is perfectly documented.
Laura-Jade Vaughan
November 23, 2025 at 22:58
OMG I literally cried reading this 🥹😭
My cousin passed away last year from HBV reactivation after her breast cancer treatment. They didn’t screen her because she’s ‘white and American’ and ‘no risk.’ She was 34. She had a toddler.
Why is this still not mandatory??? Why are we still playing Russian roulette with people’s livers???
Also, I just bought this article as a PDF and framed it. It’s going on my wall next to my ‘I survived chemo’ sticker. 💪🩺
Someone needs to make a viral TikTok about this. Like, NOW.
Jennifer Stephenson
November 25, 2025 at 07:05
Screen for HBV before immunosuppressive therapy. It’s simple. It’s cheap. It saves lives.
Do it.
Segun Kareem
November 26, 2025 at 20:40
This isn’t just about medicine-it’s about justice. In Nigeria, we don’t have the resources to screen everyone. But we do have the moral duty to try. The fact that someone in the U.S. can get a blood test and someone in Lagos might die because they can’t? That’s not healthcare disparity. That’s systemic violence.
And yet, the virus doesn’t care about borders. It doesn’t care about GDP. It wakes up when the immune system sleeps. We need global protocols. Not just guidelines. Not just suggestions. Mandates.
We are all connected. Your silence is their death sentence.
Philip Rindom
November 27, 2025 at 04:14
I get why people get frustrated with screening-it feels like overkill. But honestly? It’s not. I used to think the same until my dad got hepatitis B after a colonoscopy prep with steroids. He was fine for 30 years. Then bam. Liver failure.
Turns out, he was anti-HBc positive. We never knew.
So yeah, I get the sarcasm. But this isn’t a conspiracy. It’s biology. And biology doesn’t care if you’re busy or tired or think it’s ‘not your problem.’
Just test. Please.
Jess Redfearn
November 27, 2025 at 23:21
Wait so if I have a tattoo and I’m getting chemo, do I need to get tested? I’m confused. Is it just for Asians? What if I’m from Kansas? Do I still need it? I don’t know what any of these letters mean. HBsAg? Anti-HBc? Is that like a virus or a drug? Can someone explain like I’m five?
Ashley B
November 28, 2025 at 00:57
Of course they don’t test. The CDC doesn’t want you to know that 70% of these deaths are preventable. It’s all about money. Big Pharma doesn’t want you to know that a $20 antiviral pill could’ve saved your life. They’d rather you die and then charge you $12,000 for a liver transplant.
And the doctors? They’re complicit. They don’t want to admit they missed it. So they blame the patient. 'You should’ve known.'
Wake up. This is a cover-up. And if you’re not demanding universal screening, you’re part of the problem.
Scott Walker
November 28, 2025 at 03:41
My mom had a stem cell transplant last year. They screened her. She was anti-HBc positive. Put her on entecavir right away. She’s been cancer-free for 18 months now.
It’s not magic. It’s just… basic care.
Why is this even a debate? 🤷♂️