Contamination Testing: What It Is, Why It Matters, and What You Need to Know
When you take a pill, get an injection, or use a supplement, you expect it to do what it says—without hidden dangers. That’s where contamination testing, the process of checking pharmaceutical products for harmful substances. Also known as product purity testing, it’s the invisible gatekeeper between a medicine and your body. This isn’t just paperwork. It’s the reason your insulin doesn’t carry bacteria, your eye drops aren’t laced with mold, and your IV fluids won’t trigger a fever.
Contamination testing covers more than just dirt or dust. It checks for microbial testing, detecting bacteria, fungi, and viruses that shouldn’t be in medicine, and sterility testing, a strict lab process used for injectables and implants. It also looks for chemical residues—leftovers from manufacturing, cleaning agents, or even heavy metals that slipped through. These aren’t theoretical risks. In 2018, contaminated heparin caused over 80 deaths in the U.S. because of an unreported impurity. That’s why every batch of critical drugs must pass these tests before it leaves the factory.
It’s not just about big hospitals and fancy labs. Even over-the-counter supplements and generic drugs go through some level of contamination screening. The FDA and global health agencies require it. But not all tests are equal. Some companies cut corners. That’s why knowing what’s being checked—and what might be missed—helps you ask better questions at the pharmacy. If your medication comes with a warning about storage or expiration, that’s often tied to contamination risk. Liquid antibiotics, for example, can grow bacteria after opening. Epinephrine pens can degrade and lose potency if exposed to heat. These aren’t just storage tips—they’re contamination prevention steps you can control.
Behind every safe drug is a chain of tests: raw materials checked, production environments monitored, final products sampled and analyzed. It’s the reason you don’t hear about mass poisonings from pills every week. But it’s not perfect. Sometimes contamination slips through—like the 2012 fungal meningitis outbreak from tainted steroid injections. That’s why transparency matters. When you see a recall notice, it’s because someone caught the problem during testing. And when you read about a medication being pulled from shelves, that’s contamination testing doing its job—after the fact.
What you’ll find in the posts below isn’t just a list of articles. It’s a collection of real-world cases where contamination—or the lack of proper testing—had consequences. From expired insulin losing its power to drug interactions hiding in plain sight, these stories show why purity isn’t optional. Whether you’re a patient, caregiver, or just someone trying to make smarter health choices, understanding contamination testing helps you see beyond the label. It turns you from a passive user into an informed one.
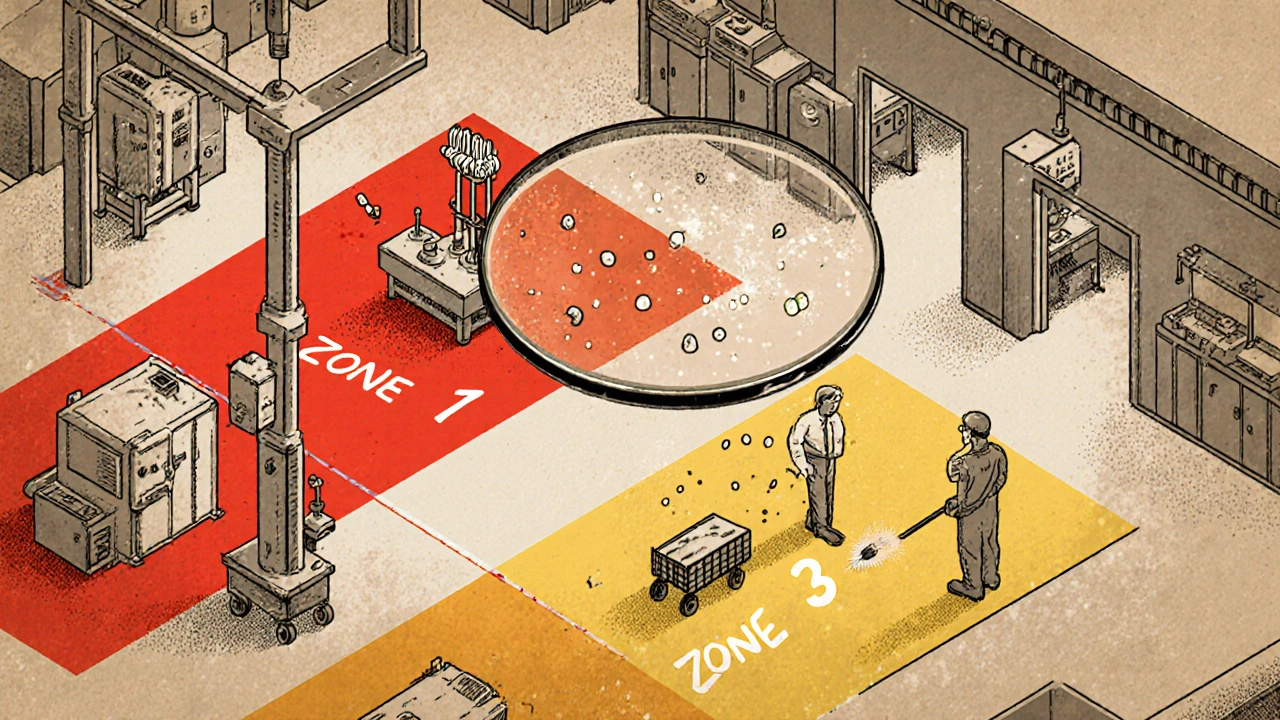
Environmental Monitoring: Testing Facilities for Contamination in Manufacturing
Environmental monitoring in manufacturing detects contamination in air, surfaces, and water before it affects products. Learn how zone-based testing, ATP swabs, and regulatory standards prevent recalls and ensure safety in pharma, food, and cosmetics.
Read More