Manufacturing Quality: What Makes a Medicine Safe and Reliable
When you take a pill, you trust it’s exactly what the label says—no more, no less. That trust comes from manufacturing quality, the set of practices and standards that ensure medicines are consistently produced to meet strict safety, strength, and purity requirements. Also known as pharmaceutical quality control, it’s the invisible system that stops contaminated, weak, or wrong drugs from reaching your medicine cabinet. This isn’t about fancy labs or expensive equipment alone—it’s about discipline, documentation, and constant checks at every step, from raw ingredients to sealed bottles.
Manufacturing quality directly connects to drug safety, how free a medication is from harmful impurities or incorrect dosing that can cause serious side effects. Think of insulin that’s lost potency or antibiotics that don’t kill bacteria because they were made under poor conditions. These aren’t hypotheticals—they’ve caused real harm. The pharmaceutical standards, the official rules set by agencies like the FDA and EMA that govern how drugs must be made exist because people have died from shortcuts. These rules cover everything: where ingredients come from, how clean the factory is, how workers are trained, and how every batch is tested before release.
It’s not just about avoiding poison. Poor manufacturing quality, the set of practices and standards that ensure medicines are consistently produced to meet strict safety, strength, and purity requirements can mean your drug simply doesn’t work. A study published in the Journal of the American Medical Association found that some generic drugs from unregulated sources had as little as 30% of the active ingredient listed on the label. That’s not a mistake—that’s negligence. And when you’re taking a drug for high blood pressure, epilepsy, or diabetes, that kind of inconsistency can be life-threatening.
Manufacturing quality also ties into medication purity, the absence of harmful contaminants like heavy metals, mold, or leftover solvents from the production process. Some pills contain trace amounts of nitrosamines—cancer-causing chemicals—because manufacturers didn’t properly control chemical reactions during synthesis. These weren’t hidden on purpose; they were missed because quality checks were skipped or rushed. The same goes for cross-contamination: if a line that makes blood pressure pills isn’t cleaned properly before making a steroid, you could end up with dangerous side effects you never signed up for.
And then there’s quality control, the ongoing testing and monitoring done at every stage of production to catch errors before the drug leaves the facility. This isn’t a one-time check. It’s hundreds of tests per batch—checking for weight, dissolution rate, chemical composition, microbial levels, and more. It’s why your prescription from a licensed pharmacy is far safer than a bottle bought from an unverified online seller. The difference isn’t marketing—it’s the system behind it.
What you’ll find in these articles isn’t theory. It’s real stories: how expired insulin became dangerous not because of age, but because of poor storage during manufacturing; how a single lab error led to a nationwide recall of blood pressure pills; how patient advocacy forced changes in how immunosuppressants are made for transplant patients. These aren’t isolated cases—they’re symptoms of a system that only works when every link is strong. Manufacturing quality isn’t something you see. But when it’s missing, you feel it. And now you know what to look for.
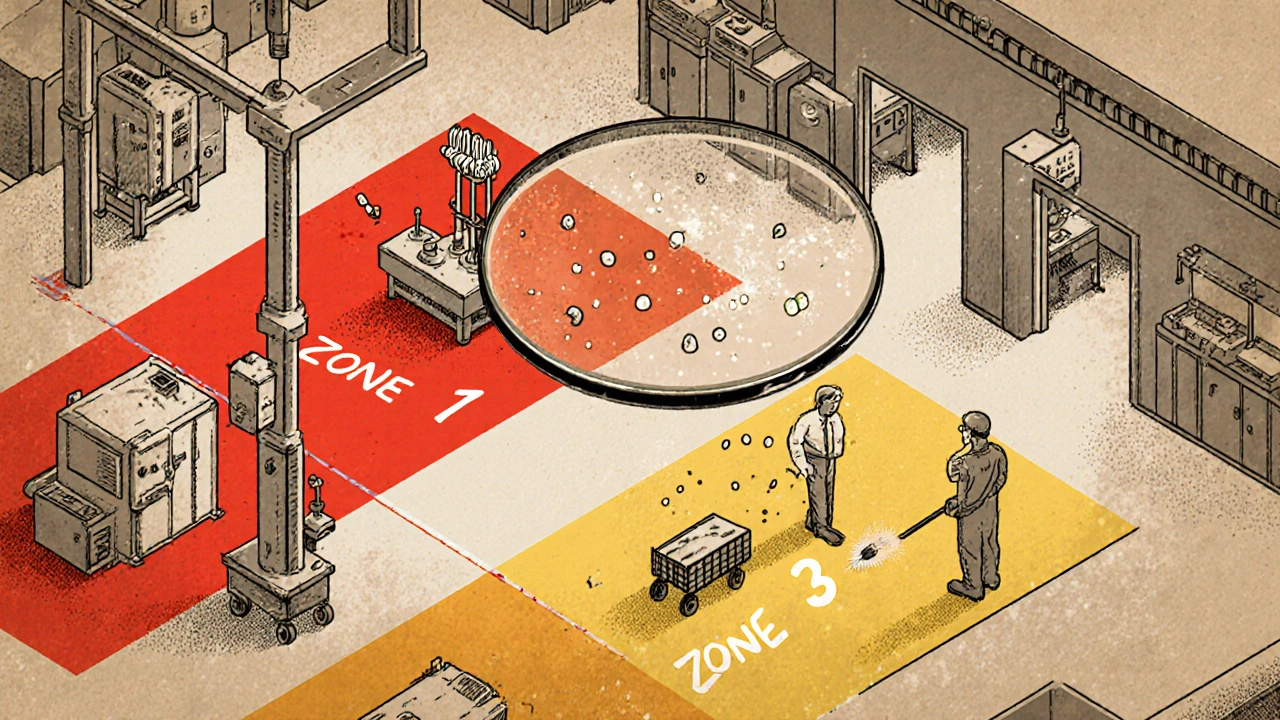
Environmental Monitoring: Testing Facilities for Contamination in Manufacturing
Environmental monitoring in manufacturing detects contamination in air, surfaces, and water before it affects products. Learn how zone-based testing, ATP swabs, and regulatory standards prevent recalls and ensure safety in pharma, food, and cosmetics.
Read More