Zocitab (Capecitabine) vs. Other Chemotherapy Options: A Practical Comparison
Zocitab vs. Other Chemotherapy Options
Zocitab (Capecitabine)
Class: Oral fluoropyrimidine
Uses: Metastatic colorectal cancer, early-stage breast cancer
Dosing: 2-week cycles (14 days on, 7 days off)
IV 5-FU
Class: Intravenous fluoropyrimidine
Uses: Standard first-line mCRC, adjuvant therapy
Dosing: Continuous infusion or bolus
S-1
Class: Oral fluoropyrimidine
Uses: East Asian populations, mCRC
Dosing: Daily oral regimen
Lonsurf (Trifluridine/Tipiracil)
Class: Novel oral agent
Uses: Refractory mCRC
Dosing: 21-day cycles
Irinotecan
Class: Topoisomerase I inhibitor
Uses: mCRC, particularly KRAS-mutant tumors
Dosing: IV, often combined with 5-FU
Decision Matrix
- Patient prefers oral therapy Zocitab, S-1, Lonsurf
- History of severe hand-foot syndrome IV 5-FU, Oxaliplatin-based
- Renal impairment S-1 (requires dose adjustment)
- Need for strong anti-tumor activity Irinotecan, Oxaliplatin
Quick takeaways
- Zocitab is an oral pro‑drug that turns into 5‑fluorouracil (5‑FU) inside tumor cells.
- It’s approved for metastatic colorectal cancer (mCRC) and early‑stage breast cancer.
- Key alternatives include IV 5‑FU, oral S‑1, trifluridine/tipiracil (Lonsurf), and combination regimens with irinotecan or oxaliplatin.
- Side‑effect profiles differ: Zocitab often causes hand‑foot syndrome, while IV 5‑FU leads to more neutropenia.
- Choosing the right drug hinges on cancer stage, previous therapies, patient lifestyle, and tolerability.
When weighing cancer treatments, the name on the bottle matters less than how the medicine works, what side effects to expect, and whether it fits a patient’s daily routine. Zocitab is a brand name for the oral chemotherapy drug capecitabine, used mainly for colorectal and breast cancers. Below we break down what makes Zocitab unique, then stack it side‑by‑side with the most common alternatives you’ll hear about in oncology clinics.
How Zocitab (Capecitabine) works
Capecitabine belongs to the fluoropyrimidine class. After swallowing the tablet, enzymes in the liver and tumor tissue convert it into 5‑fluorouracil (5‑FU), the active chemotherapeutic agent. Because the conversion happens preferentially in cancer cells, oral dosing can achieve similar tumor exposure as direct IV 5‑FU infusions, but with fewer hospital visits.
Key pharmacologic attributes:
- Bioavailability: ~70% after oral administration.
- Half‑life of capecitabine: 30-45 minutes; 5‑FU generated in tumor has a half‑life of ~10 minutes.
- Dosing schedule: 2‑week cycles (14 days on, 7 days off) are standard for mCRC; a 3‑week schedule is common for breast cancer.
When Zocitab is the right fit
Oncologists typically prescribe Zocitab for:
- Metastatic colorectal cancer (first‑line or after progression on IV 5‑FU).
- Adjuvant therapy in stageIII colon cancer when patients prefer an oral regimen.
- Early‑stage HER2‑negative breast cancer as part of a taxane‑based combination.
- Patients who want to avoid central‑line access or frequent infusion appointments.
However, not everyone tolerates Zocitab well. Hand‑foot syndrome (palmar‑plantar erythrodysesthesia) and diarrhea are the most cited dose‑limiting toxicities. If a patient has pre‑existing peripheral neuropathy, another drug may be safer.
Major alternatives to Zocitab
Below are the most frequently considered options. Each entry includes the drug’s class, typical use‑case, and a snapshot of advantages and drawbacks.
5‑Fluorouracil (5‑FU) (IV)
5‑FU is the parent compound of capecitabine. Delivered by continuous infusion or bolus, it provides predictable plasma levels but requires a peripheral line or central catheter.
- Pros: Lower incidence of hand‑foot syndrome; well‑studied dosing algorithms.
- Cons: Higher rates of neutropenia and mucositis; infusion logistics can be burdensome.
S‑1
S‑1 is an oral fluoropyrimidine used primarily in East Asia. It combines tegafur (a pro‑drug of 5‑FU) with two modulators that reduce gastrointestinal toxicity.
- Pros: Similar efficacy to capecitabine with less hand‑foot syndrome; convenient dosing.
- Cons: Limited availability in the United States; requires dose adjustment for renal impairment.
Trifluridine/Tipiracil (Lonsurf)
Lonsurf is a newer oral agent approved for refractory mCRC. It works via a different mechanism-incorporating trifluridine into DNA.
- Pros: Effective after failure of both fluoropyrimidine and irinotecan/oxaliplatin regimens.
- Cons: Higher rates of neutropenia and anemia; cost is a consideration.
Irinotecan
A topoisomerase I inhibitor given IV, often combined with 5‑FU (FOLFIRI) or capecitabine (CAPIRI).
- Pros: Strong activity in mCRC, especially in patients with KRAS‑mutant tumors.
- Cons: Diarrhea can be severe; requires pre‑medication with atropine.
Oxaliplatin
A platinum‑based IV drug, frequently paired with 5‑FU (FOLFOX) or capecitabine (CAPOX).
- Pros: High response rates in first‑line mCRC; synergistic with fluoropyrimidines.
- Cons: Cumulative peripheral neuropathy; infusion‑related hypersensitivity.
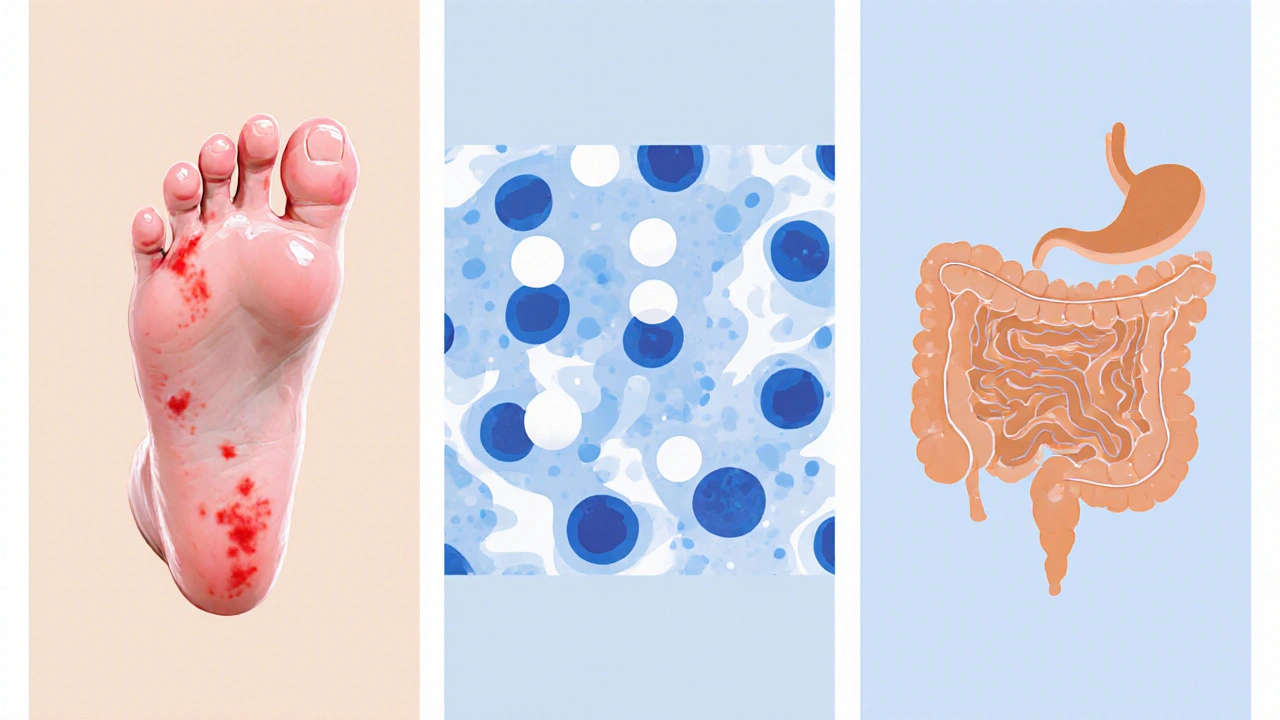
Side‑effect snapshot: Zocitab vs. alternatives
Understanding toxicity helps patients and doctors balance quality of life with tumor control.
| Drug | Hand‑foot syndrome | Neutropenia | Diarrhea | Peripheral neuropathy |
|---|---|---|---|---|
| Zocitab (Capecitabine) | 15‑30% | 5‑10% | 10‑20% | Rare |
| IV 5‑FU | 2‑5% | 15‑25% | 5‑10% | Rare |
| S‑1 | 5‑10% | 10‑15% | 8‑12% | Rare |
| Lonsurf | 3‑6% | 20‑30% | 12‑18% | Rare |
| Irinotecan‑based | 2‑4% | 10‑20% | 15‑25% | Rare |
| Oxaliplatin‑based | 1‑3% | 5‑15% | 5‑12% | 30‑40% (cumulative) |
Decision matrix: When to pick Zocitab vs. another regimen
- Patient prefers oral therapy - Zocitab, S‑1, or Lonsurf are the only oral options.
- History of severe hand‑foot syndrome - consider IV 5‑FU or oxaliplatin‑based combos.
- Renal impairment (CrCl<30mL/min) - capecitabine dose must be reduced; S‑1 may be contraindicated.
- Refractory disease after fluoropyrimidine exposure - Lonsurf offers a different mechanism of action.
- Need for rapid tumor shrinkage - combination regimens (e.g., CAPOX) provide higher response rates than single‑agent capecitabine.
In practice, oncologists blend these factors with molecular profiling (e.g., KRAS, BRAF) and patient comorbidities to craft a personalized plan.
Practical tips for managing Zocitab side effects
- Hand‑foot prevention: Use moisturizers with urea, avoid tight shoes, and report any redness early.
- Diarrhea control: Start loperamide at the first loose stool; maintain hydration with oral rehydration solutions.
- Blood count monitoring: CBC every 2weeks for the first two cycles, then before each new cycle.
- Dose adjustments: Reduce by 25% if grade2 hand‑foot persists; hold therapy for grade3 and resume at reduced dose.
These steps keep most patients on therapy long enough to see a benefit.
Frequently Asked Questions
Can I switch from Zocitab to IV 5‑FU if side effects become intolerable?
Yes. Oncology teams often transition patients who develop grade3 hand‑foot syndrome to a 5‑FU infusion schedule. The switch maintains fluoropyrimidine exposure while eliminating the oral‑related toxicity.
Is Zocitab approved for cancers other than colorectal and breast?
Capecitabine has an FDA indication for gastric cancer and for metastatic pancreatic cancer in combination with other agents, though those uses are off‑label in many centers.
How does cost compare between Zocitab and its alternatives?
Oral capecitabine is often cheaper than IV infusion regimens when you factor in infusion center fees and transportation. However, newer oral agents like Lonsurf carry a higher wholesale price, which can offset convenience.
What monitoring is required during Zocitab therapy?
Baseline CBC, liver panel, and renal function are taken before starting. CBC is repeated every 2weeks for the first two cycles, then before each new cycle. Dermatologic exams for hand‑foot syndrome are done at each office visit.
Can Zocitab be used together with targeted therapies?
Yes. In colorectal cancer, capecitabine is combined with bevacizumab (an anti‑VEGF antibody) or with EGFR inhibitors in KRAS‑wild‑type disease. In breast cancer, it’s paired with trastuzumab for HER2‑positive tumors.
Bottom line
Choosing between Zocitab and other chemotherapy options isn’t a one‑size‑fits‑all decision. If you value an oral regimen and can manage hand‑foot syndrome, Capecitabine alternatives like S‑1 or Lonsurf may be worth a look, but traditional IV 5‑FU or combination regimens often provide a safety net when side effects pile up. Talk with your oncologist about disease stage, prior treatments, and lifestyle preferences - that conversation will steer you to the regimen that balances efficacy with tolerability.





11 Comments
Warren Workman
October 3, 2025 at 02:30
While the comparative matrix is well‑structured, one must interrogate the underlying pharmacokinetic assumptions that drive the dosing schedules; the oral bioavailability figures quoted (~70%) oversimplify inter‑patient variability, especially in populations with hepatic enzyme polymorphisms. Moreover, the emphasis on hand‑foot syndrome prevalence neglects the cumulative neurotoxicity profile associated with downstream metabolite accumulation. From a mechanistic standpoint, capecitabine’s conversion cascade introduces an additional enzymatic checkpoint that can be a double‑edged sword in terms of therapeutic index. Clinicians should therefore calibrate dose‑dense regimens against real‑world toxicity databases rather than relying solely on trial‑derived percentages. In practice, the “one‑size‑fits‑all” paradigm rarely holds true.
Kate Babasa
October 13, 2025 at 12:30
The presented side‑effect tableau, while comprehensive, also invites a nuanced discussion; clinicians must weigh hand‑foot syndrome against neutropenia, especially when patients exhibit pre‑existing dermatologic sensitivities, and consider the convenience of an oral regimen versus the logistical demands of continuous infusion, all within the broader context of personalized medicine, which inherently demands a balance between efficacy, tolerability, and quality of life; consequently, the decision matrix serves as a valuable scaffold, yet it should not eclipse individualized risk‑benefit analyses.
king singh
October 23, 2025 at 22:30
The oral route indeed reduces the logistical burden for many patients.
Adam Martin
November 3, 2025 at 07:30
I have to say, the enthusiasm surrounding an “easy‑pill” solution is almost intoxicating, as if pharmacology were reduced to a vending‑machine transaction.
Capecitabine’s allure lies in its promise to replace a dreaded IV line with a daily tablet, yet the underlying biochemistry remains a labyrinth of enzymatic conversions.
First, the pro‑drug must survive gastric acidity, be absorbed, then traverse hepatic and tumor‑specific enzymatic pathways before finally releasing the cytotoxic 5‑FU moiety.
Each step introduces variability that clinical trials can only approximate, not fully capture.
Second, the side‑effect spectrum, particularly hand‑foot syndrome, is not merely a statistical footnote but a dose‑limiting toxicity that can cripple a patient’s daily function.
When you compare that to IV 5‑FU, which, despite its logistical challenges, offers more predictable plasma kinetics, the trade‑off becomes less obvious.
Third, the cost considerations, especially with newer oral agents like Lonsurf, can strain healthcare budgets and patient out‑of‑pocket expenses.
Insurance formularies often hinge on negotiated pricing, turning clinical preference into a bureaucratic battleground.
Moreover, the decision matrix presented assumes a binary choice between oral and IV, ignoring hybrid regimens that blend the two for synergistic effect.
In reality, many oncologists employ CAPOX or FOLFIRI, capitalizing on the pharmacodynamic complementarity of fluoropyrimidines with platinum or irinotecan backbones.
These combinations, while more complex, have demonstrated superior response rates in metastatic colorectal cancer cohorts.
The article also glosses over the impact of renal impairment on drug clearance, a factor that can dramatically alter dosing schedules.
Patients with reduced creatinine clearance may experience heightened toxicity, necessitating vigilant monitoring and dose adjustments.
Furthermore, the narrative neglects the psychological dimension of oral chemotherapy adherence, a non‑trivial component of therapeutic success.
Missed doses, intentional or accidental, can erode treatment efficacy and foster resistance.
In sum, while the convenience of an oral pill is undeniably attractive, the underlying pharmacologic intricacies, economic constraints, and patient‑specific factors demand a far more circumspect approach than the table suggests.
Ryan Torres
November 13, 2025 at 17:30
Don't be fooled by the glossy charts – the pharma giants are pushing capecitabine because they want a blockbuster pill that ties patients to a lifelong subscription, and the subtle hand‑foot toxicity is just a clever way to keep you on the drug longer while the real cures get buried in red tape 🚨😠. The side‑effect percentages are manipulated, the trials are cherry‑picked, and the push for oral administration is a data‑driven strategy to cut hospital revenue streams, which ultimately benefits the shareholders, not the patients 💸. Keep an eye on the regulatory filings; they reveal a pattern of underreporting severe neuropathy in favor of marketing hype. This isn’t just medicine, it’s a profit‑first agenda masquerading as patient‑centric care.
shashi Shekhar
November 24, 2025 at 03:30
Sure, the matrix looks polished, but let’s be honest – it’s just a pretty spreadsheet that glosses over the gritty reality of side‑effects, and who really has the energy to dissect every nuance when you’re already juggling chemo appointments?
Marcia Bailey
December 4, 2025 at 13:30
Great job breaking down the key differences! 🎉 For anyone navigating these options, remember to discuss dosing schedules and side‑effect management with your oncology team, and don’t hesitate to ask about supportive care resources – they can make a huge difference in quality of life 😊.
Dhananjay Sampath
December 14, 2025 at 23:30
Ryan, your concerns about pharmaceutical motivations are certainly noted, and while the profit motive is an undeniable factor in drug development, it’s also true that rigorous clinical trials and regulatory oversight aim to balance efficacy with safety; therefore, a nuanced view that acknowledges both economic drivers and genuine therapeutic advancements may serve patients best.
kunal ember
December 25, 2025 at 09:30
When evaluating capecitabine alongside its intravenous counterpart, it is essential to consider not only the pharmacodynamic equivalence but also the pharmacokinetic variability introduced by gastrointestinal absorption, hepatic conversion enzymes such as carboxylesterase and thymidine phosphorylase, and the subsequent intracellular activation to 5‑fluorouracil; these biochemical pathways can lead to inter‑patient differences in plasma concentrations, which in turn affect both therapeutic response and the incidence of adverse events like hand‑foot syndrome, thereby necessitating individualized dose adjustments based on renal function and performance status, especially in elderly cohorts where comorbidities may further complicate the clinical picture.
Kelly Aparecida Bhering da Silva
January 4, 2026 at 19:30
It’s infuriating how foreign pharmaceutical conglomerates continue to dominate our cancer treatment landscape, pushing expensive oral agents like capecitabine while sidelining home‑grown research that could develop more affordable, culturally appropriate therapies; we must demand greater investment in domestic oncology innovation and stricter scrutiny of imported drugs that may not align with our national health priorities.
Michelle Dela Merced
January 15, 2026 at 05:30
Honestly, Kelly, this feels like a blockbuster drama – the “Big Pharma vs. Our Nation” storyline is so over‑the‑top 😱! While I get the frustration, let’s keep the conversation grounded and focus on evidence‑based choices rather than turning it into a melodramatic showdown 🙄.