Switching Generics: What You Need to Know Before You Switch
When you hear switching generics, the practice of replacing a brand-name drug with a chemically identical generic version. Also known as generic substitution, it’s one of the most common ways to cut prescription costs without losing effectiveness. But here’s the thing: not all generics are created equal, and switching isn’t always as simple as swapping one pill for another.
Some people switch without a second thought—maybe their pharmacy did it automatically, or their insurance pushed them toward the cheaper option. But if you’re on a drug with a narrow therapeutic index—like warfarin, thyroid meds, or seizure drugs—even tiny differences in how your body absorbs the generic can cause real problems. That’s why drug equivalence, how closely a generic matches the brand-name version in how it works in your body. Also known as bioequivalence, it’s the science behind whether a switch is safe. The FDA says generics must be within 80–125% of the brand’s absorption rate. Sounds close, right? But for some people, that small window is enough to throw off their INR, trigger seizures, or make their blood pressure spike.
Then there’s the pharmacy substitution, when a pharmacist swaps your prescription for a different generic without asking. Also known as automatic substitution, it’s legal in most places—but you don’t have to accept it. You can ask for the brand, or even the specific generic brand you’ve been using. If your doctor thinks a certain version works better for you, they can write "dispense as written" or "do not substitute" on the script. That stops the pharmacy from switching without your consent.
And don’t forget about generic drug substitution, the broader process of changing from one generic to another. Also known as generic-to-generic switching, it’s happening more often as insurers juggle multiple generic suppliers. One month you get the generic from Company A, next month it’s Company B. They’re both FDA-approved, but different fillers, coatings, or manufacturing methods can change how the drug behaves in your body. People on blood thinners, epilepsy meds, or psychiatric drugs report mood swings, dizziness, or breakthrough symptoms after these switches—even though the active ingredient is the same.
That’s why the posts below cover real cases: how a switch to a different generic version of warfarin spiked INR levels, why insurance sometimes denies coverage for certain generics, how combination blood pressure pills are made, and what to do when your pharmacy switches your meds without telling you. You’ll also find guides on how to appeal denials, how to read your prescription label for hidden changes, and when to push back—because your health isn’t a cost-saving experiment.
Switching generics isn’t always bad. For many, it’s a smart, safe way to save money. But it’s not risk-free. The key is knowing when to ask questions, when to insist on staying put, and how to spot when something’s off after a switch. The information below will help you do exactly that—without the jargon, without the fluff, just what you need to stay in control of your meds.
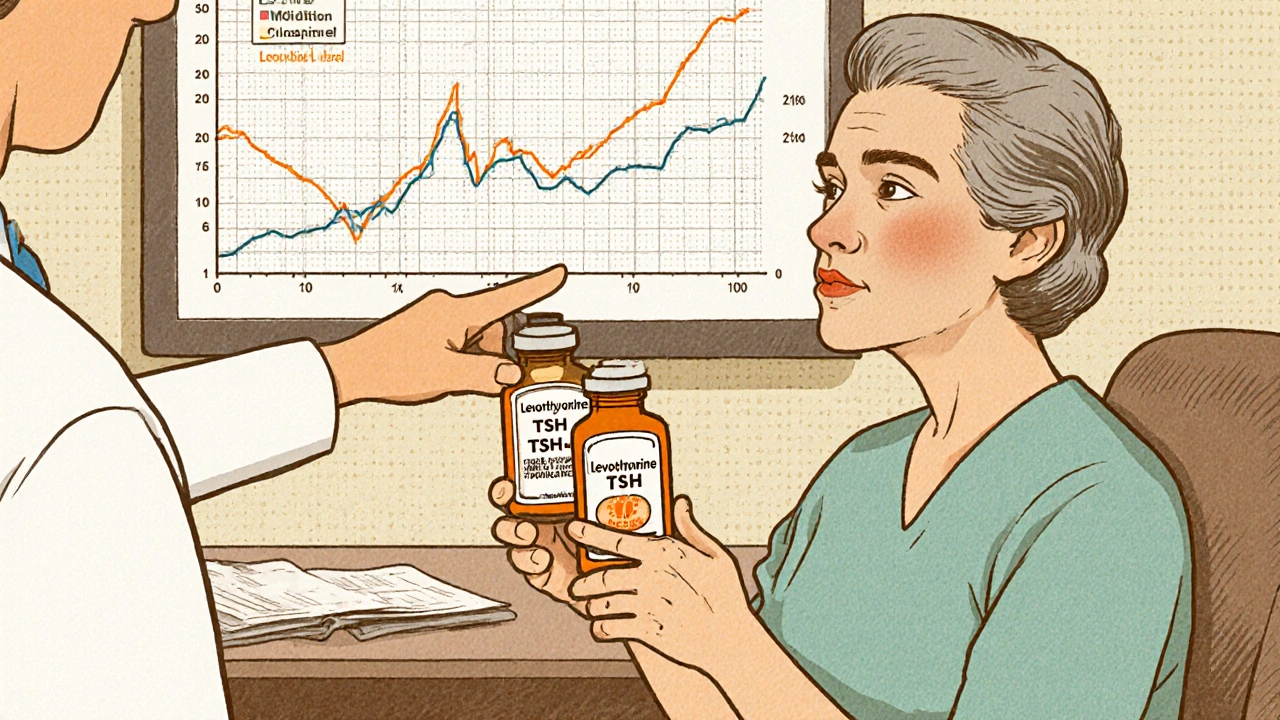
When Doctors Adjust Doses After Switching to Generic Medications
Switching to generic medications can be safe-but not for all drugs. For narrow therapeutic index drugs like levothyroxine and warfarin, even small changes in formulation can require dose adjustments. Here’s what you need to know.
Read More
When Doctors Adjust Doses After Switching to Generic Medications
Switching to generic medications can be safe-but for certain drugs like warfarin, levothyroxine, and phenytoin, even small changes can cause serious side effects. Learn when dose adjustments are needed and how to protect yourself.
Read More